Nuvaxovid
After the approval of the mRNA vaccines Corminaty BiontechPfizer Spikevax Moderna and the vector-based vaccines Vaxzevria Astra Zeneca and Covid-19 Vaccine Janssen a further. Data från Australien pekar mot en ökad.
Nuvaxovid The New Subunit Sars Cov 2 Vaccine Mci Innsbruck
No hasta el momento todo menor de 18 años esta.
. 2 Xinhua -- Nuvaxovid the COVID-19 vaccine created by US. Det proteinbaserade covid-19-vaccinet Nuvaxovid inte ska ges till personer som är 30 och yngre meddelar Folkhälsomyndigheten. Vial and carton labels with English-only labelling.
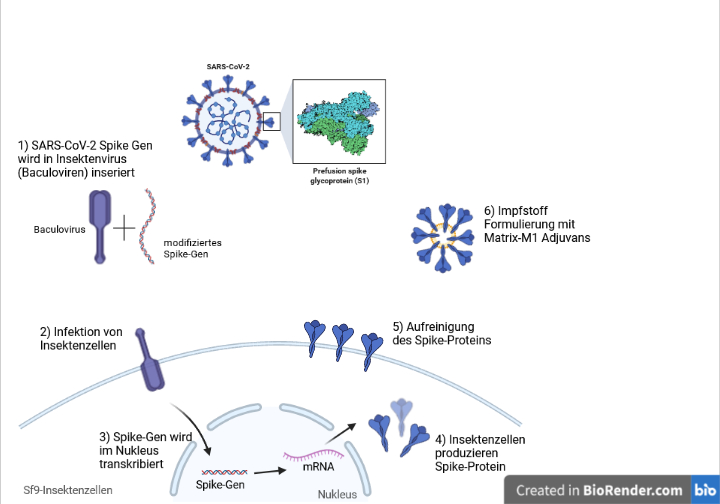
Nuvaxovid Novavax is approved and available for use as a primary course in people aged 12 years and over. 11 hours agoThe US company Novavax came up with another vaccine to fight the virus - Nuvaxovid. About Nuvaxovid NVX-CoV2373 Nuvaxovid is a protein-based vaccine engineered from the genetic sequence of the first strain of SARS-CoV-2 the virus that causes.
The Technical Advisory Group for Emergency Use Listing listed Nuvaxovid NVX-CoV2373 vaccine against COVID-19 and Covovax NVX-CoV2373 vaccine against COVID-19. The Summary of Product Characteristics is a description of a. Nuvaxovid is the first protein-based COVID-19 vaccine granted.
Nuvaxovid offers a high level of protection against COVID-19 which is a critical need in the current pandemic. The Novavax Nuvaxovid COVID-19 vaccine was authorized for use in Canada under the Food and Drug Regulations. This is a multidose vial.
Nuvaxovid dispersion for injection. Nu stoppar Folkhälsomyndigheten användningen bland personer som är 30. Novavax is approved and available for use as a booster in.
The Nuvaxovid vaccine a protein-based vaccine engineered from the genetic sequence of the first strain of the SARS-CoV-2 virus which causes COVID-19. Company Novavax should not be given to individuals. Nuvaxovid COVID-19 vaccines are available for use in the United Kingdom as of September 27 2022.
This vaccine is currently being used in Sweden and as of date a total of 7000. Det eftersom att data från. The Nuvaxovid NVX-CoV2373 Novavax vaccine is a recombinant spike S protein nanoparticle vaccine combined with the Matrix-M adjuvant.
1 day ago3rd November 2022 0355 GMT11. On December 20 2021 the. Nuvaxovid vaccine pause for young people justice system spending Västerås shooting young women have more debt than 10 years ago.
The World Health Organization issued an emergency use listing EUL for Nuvaxovid TM following its assessment and approval by the European Medicines Agency. Folkhälsomyndigheten rekommenderar att det proteinbaserade covid-19-vaccinet Nuvaxovid inte ges till personer som är 30 år och yngre. Qualitative and quantitative composition.
Contact your healthcare professional if you have any questions about the product. Company Novavax should not be given to individuals. Information about the COVID-19 vaccine Nuvaxovid approved by the MHRA on 03 February 2022.
1 Menores de 18 años requieren certificado de vacunación. 1 day agoSverige Covid-19-vaccinet Nuvaxovid skulle erbjudas till personer som var tveksamma till vaccinationen. Find detailed technical information such as the product monograph and.
Nuvaxovid is composed of purified full length severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation. COVID-19 Vaccine recombinant adjuvanted 2. Beslutet är temporärt och gäller från.
1 day agoBakgrunden till beslutet är signaler om ökad risk för hjärtmuskelinflammation myokardit och hjärtsäcksinflammation perikardit. Name of the medicinal product. 1 day agoPublicerad idag 0702.

Ema Plans Anaphylaxis Label On Novavax Covid 19 Vaccine

Nuvaxovid Fifth Vaccine Against Covid Authorised In Eu Euractiv Com

Nuvaxovid Covovax Novavax Vaccine Covid 19 Info Vaccines
Switzerland Approves Novavax S Covid Vaccine For 12 18 Year Olds Swi Swissinfo Ch

Say What You See Novavax Taps Its Own Branding For Latest Covid Vaccine Fierce Pharma

My Kingdom For A Booster Shot Hiccups Are Yet Another Vaccine Trial Hassle Medpage Today
Novavax Nuvaxovid Covid 19 Vaccine Approved In South Korea For Use In Adolescents Aged 12 Through 17 World Pharma Today
Fda Authorizes Novavax Covid 19 Vaccine For Emergency Use In Us Abc News

Ema Recommends Nuvaxovid For Authorisation In The Eu Certifico Srl

Cdc Panel Backs Novavax Covid Vaccine For Adults Medpage Today

Novavax Covid 19 Vaccine Nuvaxovid Data On Side Effects
U S Fda Authorizes Novavax Covid Vaccine For Adults Reuters

Nvx Cov2373 Recombinant Adjuvanted Covid 19 Vaccine

Novavax S Covid 19 Vaccine Approved For Canadians 18 And Older Cbc News

Is The Novavax Covid Vaccine Worth The Hype Medpage Today

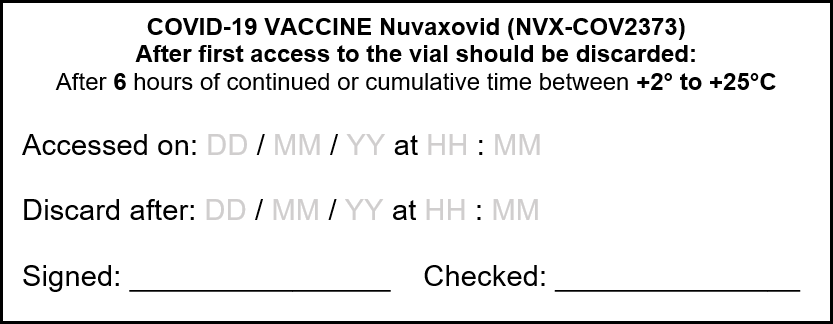
Management Of Covid 19 Vaccine Nuvaxovid Novavax From Refrigerator To Administration Vaccination
Fda Committee Oks Novavax S Late To The Game Covid 19 Vaccine Cbs News

Novavax Covid 19 Vaccine Nuvaxovid Approved By Mhra Gov Uk
Novavax Covid 19 Vaccine Nuvaxovid Approved By Mhra Pharmatutor